eQMS for GDP Logistics
With BizzMine you have a validated and compliant software for your QMS.
Monitor, measure, and improve the quality of your distribution practices.
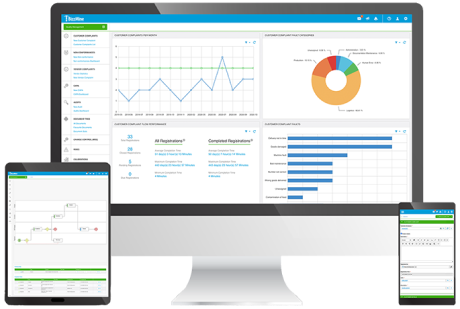

A centralized platform helps you ensure compliance with specific GDP regulations and guidelines.

Increased efficiency
Automate and link all quality processes: document control and management of change, CAPA, risks, complaints, audits...
Facilitate collaboration among stakeholders, such as quality assurance, manufacturing, and regulatory affairs teams.
Analytics and reporting help you identify trends in quality data and continuously improve processes.
Real-time visibility lets you quickly identify issues and prevent negative impacts on product safety and efficacy.
Register your data on any device: laptop, tablet or smartphone. Go for cloud or on-premises.
"BizzMine has had a considerable impact on our GDP compliance. The software has made the entire process more streamlined and efficient. Now, all departments are using BizzMine for full quality management across all functions, and it has transformed our quality culture."
Jenna Cordery - Geodis FF UK LTD
See BizzMine in action
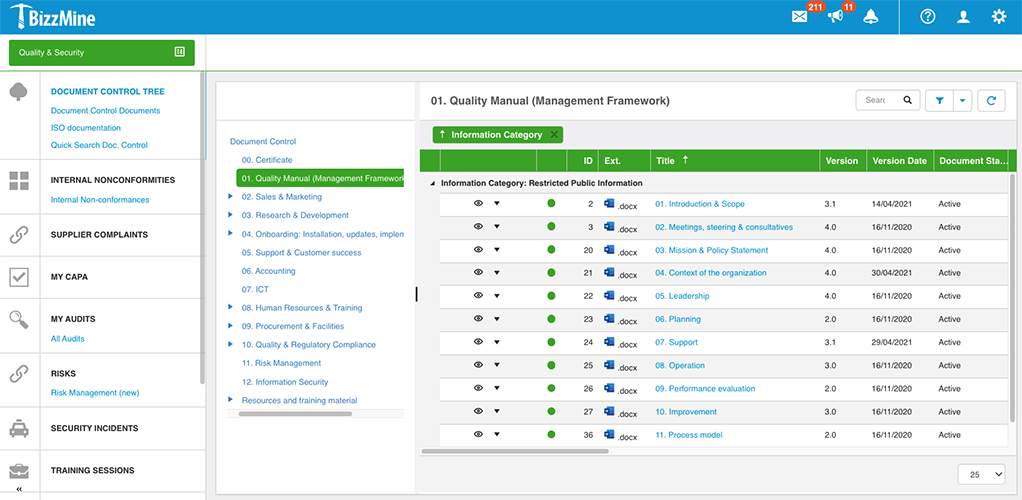
Document Control
Digitize documents and keep track of written procedures. Review and approve instruction documents easier and faster than before.
Stay up to date on the latest document versions you can use.
Choose the layout of your digital forms at each step of the workflow process.
The exchange of general instructions and changes now happens automatically and hence very fast. BizzMine is of great importance to us, especially because we work on different sites.

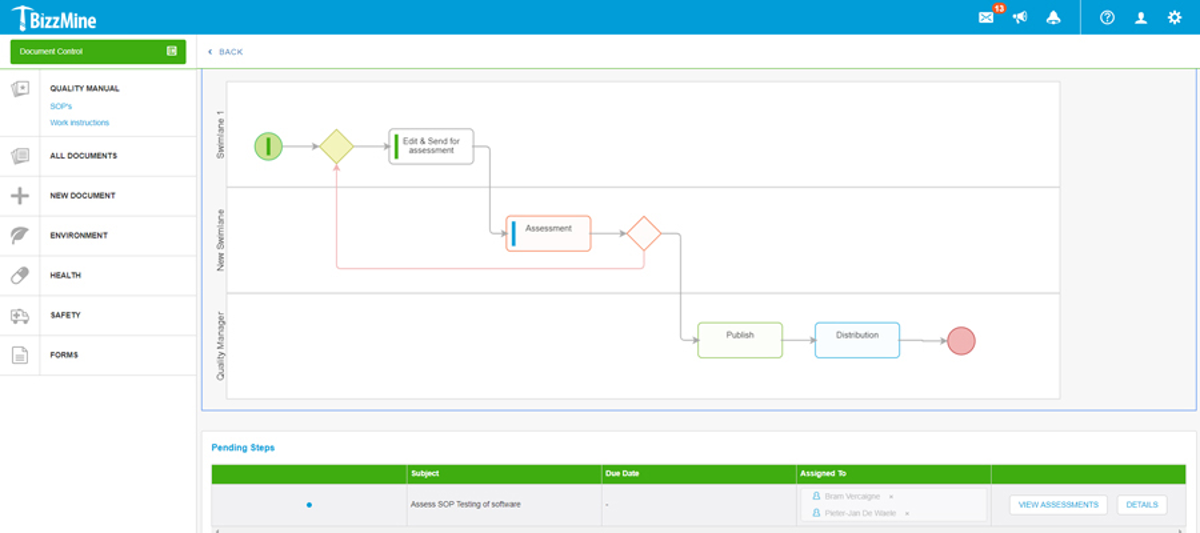
Audit Management
Be prepared for your next internal or external audit.
With BizzMine, you can easily view the required work instructions.
Identify, analyze, and track all possible deviations that occurred during the audit.

BizzMine allows us to integrate a controlled system for improved document and audit management. It also made it easier for us to achieve our ISO standards.
CAPA Management
Launch and link CAPAs from any other process. Initiate CAPAs to correct and prevent deviations in accordance with quality risk management principles.
Customizable dashboards make it easy to present the most valuable and useful data.

Risk Management
Automate your risk management process.
Mitigating risk is more critical to a GDP-compliant quality system than ever before.
Identify potential high-risk areas allowing the management to take appropriate preventive action and reduce wasting resources on low-risk areas.

Discover BizzMine now
Complaint Management


Calibration Management
Organize calibration processes in one place.
Capture all relevant data in the customizable calibration form and attach scans or images to document the results.
Schedule your calibration work and rely on the intuitive workflow process for deadlines.

Non-conformances
Streamline quality processes to manage non-conformities.
Deviations from established procedures are documented and investigated.
You can easily set up a workflow to handle deviations and defects.
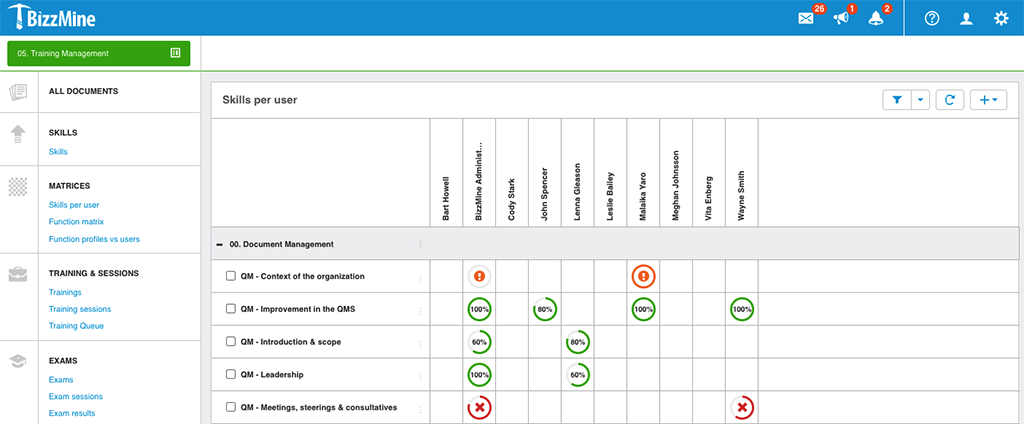
Training Management
Manage all data on training, skills, and exams in one central database.
Get a color-coded overview of user and function skills in the skills matrix. And link training, skills, and exams to documents.
We start our day with BizzMine and if we follow the correct procedure, there is no way we can go on working with a wrong or outdated document.
As we also work with medical goods, it is crucial for us to work with the correct versions of documents. We are strictly bound to the GDP regulations and have to follow strict rules regarding documentation. With BizzMine we can be sure that we meet these requirements.
















